Silky Cup Size - L
Silky Cup Size - M
Silky Cup Size - S
Silky Cup Size - M + L (Pack of 2 Menstrual Cups)
Silky Cup: A Really Safe "Clinically Tested" Reusable Menstrual Cup.
Menstruation is a normal, healthy part of a woman's life.
Reusable menstrual Silky Cup for girls and women of all ages and flow levels.
The menstrual Silky Cup can be reused up to 5 years.
Silky Cup is the safest menstrual cup in the world for feminine hygiene; Silky Cup has proven its quality and durability on all tests.
A Silky Cup is a bell-shaped device worn inside the vagina during menstruation to collect menstrual fluid. Menstrual Silky Cup provides a viable alternative to sanitary napkins, tampons, sanitary towels, sanitary pads, cloth menstrual pads, panty liners and disposable sanitary pads. The reusable menstrual Silky Cup is the most attractive alternatives to other feminine hygiene products.
Reusable menstrual Silky Cup gives you 12-hours wear time as well as comfort and convenience during your period. Silky Cup has a life expectancy of up to 5 years, if maintained correctly. Try it and you'll see how it fits into your busy life! It is the most modern intimate hygiene product for girls and women.
A menstrual Silky Cup offers many advantages such as sleeping well, visiting the pharmacy less, holds more liquid, wards off rashes, lightens your purse, requires less changing, saves money, allows for an extra five hours in between changes, cause you to suffer fewer leaks. Silky Cup can easily manage heavy bleeding, irregular periods, prolonged menstrual periods and perimenopause.
Silky Cup provides 12 hours leak free protection. Silky Cup offers up to 12 hours of hassle-free day and night menstrual care and covers your heaviest to lightest menstrual flow. Silky Cup is ideal for women of all shapes and sizes. Silky Cup keeps you clean, dry and super active throughout periods. Ideal for all daily activities such as playing sports, travelling, swimming, cycling, diving, trekking, caving, dancing, climbing, bowling, walking, driving, sitting and sleeping; now no more odors, wetness, stains, itching or leaking during periods. Hole-less Menstrual Silky Cups are designed for higher capacity, so now you don't need to awake till midnights. No more leakage; even you don't feel its presence! Periods care made easy. Travel, exercise, play sports, dance and swim with no fear of leaks during your periods. Silky Cup can hold up to 30 ml discharge during periods, which is 3 times more than tampons or pads can absorb. Silky Cup is easy to insert, remove and use. Silky Cup is a reusable and washable feminine hygiene product.
Silky Cup is a more sanitary feminine hygiene solution than sanitary napkins, tampons or pads. While using a Silky Cup, even you do not feel its presence. Silky Cup can be worn overnight. Silky Cup is comfortable and does not cause irritation or dryness. Silky Cup has no wings or strings. It contains no latex, silicone, PVC, Phthalates, proteins, BPAs, dioxins, bleaches, perfumes, pesticides or carcinogens. Silky Cup is non-allergenic and non-toxic. It leaves no residual fibers.
Now it is time to try a Silky menstrual Cup. Menstrual Silky Cup helps you to manage your periods. Menstrual Silky Cup is easily creates a seal inside to experience a complete leak free period. The menstrual Silky Cup enhances the quality of a woman's life. You will realize those period days just like any other normal days. Using a menstrual Silky Cup can change your life for the better. One-time investment that saves your valuable time and money. Reusable menstrual cup is also known as Vaginal Cup, Menses Cup, Menstruation Cup, Masikdharm Cup, Period Cup, Sanitary Cup, Tampon Cup, Masik Cup, Masik Dharm Cup, Silicone Cup for periods and Silicone menstrual cup.
Silky Cup sizes
Size L - Suitable for women who have tall and strong build or given birth to a child by vaginally or by caesarean section or who are over the age of 30 years.
Size M - Suitable for women who have medium build or not given birth to a child or women up to the age of 30 years.
Size S - Suitable for preteen girls and very slim or small framed teenage girls up to the age of 15 years.
Capacity
Silky Cup Size L : 30 ml, Size M : 24 ml, and Size S : 18 ml.
Safety, Quality and Standards
We believe that all women should have a happy and healthy period experience. That is why we do everything we can to ensure our products are tested and manufactured with women’s health and comfort in mind.
The Silky Cup is tested within International standards. ISO 10993 is a Quality Standard governed by the International Organization of Standardization in Geneva, Switzerland, and is recognized and practiced in over 160 countries around the world. ISO 10993 is the quality standard for medical devices.
Silky Cup is safe; Silky Cup is developed especially for menstrual hygiene management. Silky Cup has been tested as per United States pharmacopeia (USP) and International Organization for Standardization (ISO) International standards. Silky Cup has passed the all necessary ISO 10993 tests with great success.
“Silky Cup is tested according to ISO 10993-5 (Cytotoxicity), ISO 10993-10 (Mucosal Irritation), ISO 10993-10 (Sensitization), Directive 2011/65/EU Restriction of Hazardous Substances Directive (ROHS), USP <88> “CLASS VI” Tests Acute Systemic Toxicity , Intracutaneous Irritation and Intramuscular Implantation.”
Sizing and selection
Silky Cup has a larger, a medium and a smaller size. The larger size is normally recommended for women who are over 30, have given birth vaginally, or have an unusually heavy flow. The medium size is normally recommended for women under 30, who have not given birth vaginally, as well as menstruating women and girls who are more physically fit, as those with stronger pelvic floor muscles may find a larger cup uncomfortable. The Silky Cup with the smallest size diameter is recommended for preteen girls and very slim or small framed teenage girls, Capacity is important to women who have a heavier flow; however, all of the Silky Cups currently available have a larger capacity than a regular tampon and pads.
Use
The Silky Cup is first folded or pinched, and then inserted into the vagina. It will normally unfold automatically and create a light seal against the vaginal walls. In some cases, the user may need to twist the cup or flex the vaginal muscles to ensure the cup is fully open. In most cases, a Silky Cup will migrate upwards and sit against the cervix. If correctly inserted, the cup should not leak or cause any discomfort, those who are familiar with inserting a non-applicator tampon should learn faster how to insert a cup, though there is still a learning curve. There are a number of different folding techniques that can be used for insertion. If lubrication is necessary for insertion, it should be water-based,
After about 4–12 hours of use (depending on the amount of flow), the cup is removed by reaching up to the stem of the cup in order to find the base. Simply pulling on the stem is not recommended to remove the Silky Cup, as pulling it down will create suction. The solid base of the cup is pinched to release the seal, and the cup is removed. After emptying, a reusable Silky Cup should be rinsed or wiped and reinserted. It can be washed with a mild soap, and sterilized in boiling water for a few minutes at the end of the cycle. Alternatively, sterilizing solutions (usually developed for baby bottles and breast pump equipment) may be used to soak the Silky Cup.
Acceptability studies
A randomized controlled feasibility study has been conducted among adolescent primary school girls in rural western Kenya, providing menstrual cups or sanitary pads over traditional menstrual care items, such as cloth or tissue. After six months of provision, researchers reported that menstrual cup users were free from embarrassing leakage, odor, and could engage in class activities and sport without humiliation and teasing.[2]
Costs
One Silky Cup is usually more expensive than a package of sanitary napkins or tampons. However, Silky Cups can be used for many years, which makes them more favourable compared to tampons and disposable pads in the longer term. Depending on the female's cycle and habits, within about 3 to 6 months a Silky Cup can start to save money compared to buying pads or tampons
References
- Howard C, Rose CL, Trouton K, Stamm H, Marentette D, Kirkpatrick N, Karalic S, Fernandez R, Paget J (June 2011). "FLOW (finding lasting options for women): Multicentre randomized controlled trial comparing tampons with menstrual cups".
- Mason L; Laserson K. F.; Oruko K.; Nyothach E.; Alexander K. T.; Odhiambo F. O.; Eleveld A.; Isiye E.; Ngere I.; Omoto J.; Mohammed A.; Vulule J.; Phillips-Howard P. A. (2015). "Adolescent schoolgirls' experiences of menstrual cups and pads in rural western Kenya: a qualitative study".
Clinical Studies
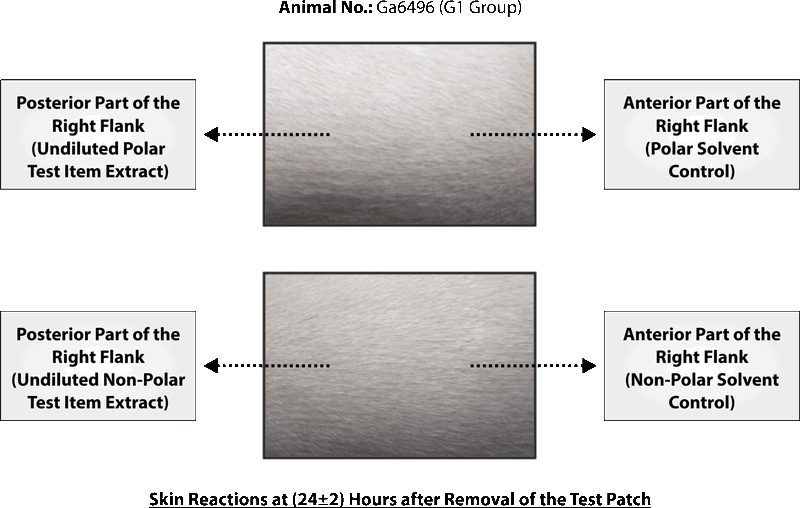
STUDY TITLE: SKIN SENSITIZATION STUDY OF SILKY CUP (MENSTRUAL CUP) IN GUINEA PIGS (MAXIMIZATION TEST)
RESULTS AND DISCUSSION
Clinical Signs of Toxicity and Mortality
No clinical signs of toxicity and mortality were observed in any of the animals in all the groups.
PHOTOGRAPHS OF CHALLENGE PHASE
CONCLUSION
Based on the results of the experiment, it can be concluded that polar and non-polar extracts of test item Silky Cup (Menstrual Cup) was found to be “Non-sensitizer” to the skin of the Guinea pigs under the experimental conditions and doses employed as per ISO 10993-10: 2010(E).
STATEMENT OF GLP COMPLIANCE
The Study No.: BIO-TX 2110, entitled “Skin Sensitization Study of Silky Cup (Menstrual Cup) in Guinea Pigs (Maximization Test)” was performed in compliance with the OECD Principles of Good Laboratory Practice [C(97)186/Final] and in accordance with FDA Good Laboratory Practice 21 CFR Part 58.
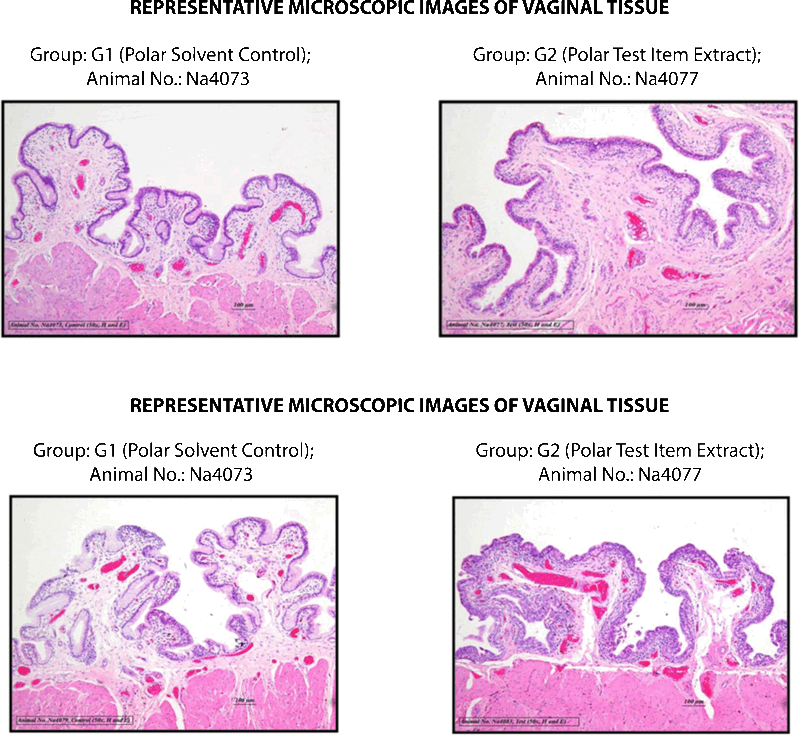
STUDY TITLE: VAGINAL IRRITATION TEST OF SILKY CUP (MENSTRUAL CUP) IN NEW ZEALAND WHITE RABBITS
RESULTS AND DISCUSSION
Clinical Signs of Toxicity and Mortality
No treatment related clinical signs of toxicity and mortality were observed in any of the group animals.
CONCLUSION
Based on the observed results of the experiment and under the experimental conditions employed, the irritation index of both polar and non-polar extracts of Silky Cup (Menstrual Cup) was observed less than one and hence the test item was considered as “non-irritant” to the vagina of New Zealand white rabbit when administered into the vagina for five consecutive days.
STATEMENT OF GLP COMPLIANCE
The Study No.: BIO-TX 2111, entitled “Vaginal Irritation Test of Silky Cup (Menstrual Cup) in New Zealand White Rabbits” was performed in compliance with the OECD Principles of Good Laboratory Practice [C (97)186/Final] and in accordance with FDA Good Laboratory Practice 21 CFR Part 58.
STUDY TITLE: IN VITRO CYTOTOXICITY STUDY OF SILKY CUP (MENSTRUAL CUP) BY ELUTION METHOD
RESULTS AND DISCUSSION
L-929 mouse fibroblast cells of sub confluent monolayer were treated with test item, negative control, positive control and blank in triplicates were incubated for 48 hours at 37°C and 5% CO2. Post treatment with extracts of test item, positive control, negative control and blank, L-929 mouse fibroblast cells were examined microscopically for any changes in cell morphology and cell lysis. L-929 mouse fibroblast cells treated with the test item extract showed discrete intracytoplasmic granules, no cell lysis, no reduction of cell growth (Grade 0). Since there was no reactivity and the grade is not greater than 2, the test item is considered as non-cytotoxic. Cells in the blank wells and negative control showed discrete intracytoplasmic granules, no cell lysis, no reduction of cell growth and no reactivity (Grade 0), whereas the positive control showed nearly complete or complete destruction of the cell layers with severe reactivity (Grade 4).

CONCLUSION
Based on the results obtained under laboratory testing conditions, the test item, Silky Cup (Menstrual Cup) is considered as non-cytotoxic to the subconfluent monolayer of L-929 mouse fibroblast cells.
The Study No. BIO-GT 532, entitled “In vitro Cytotoxicity Study of Silky Cup (Menstrual Cup) by Elution Method” was performed in compliance with the OECD Principles of Good Laboratory Practice [C(97)186/Final] and in accordance with FDA Good Laboratory Practice 21 CFR Part 58.
What is USP Class VI?
The United States Pharmacopoeia (USP) is an independent organisation that established a set of standards to ensure the quality of medicines and health care technologies. Many device manufacturers continue to use the USP Class VI test to determine biocompatibility though ISO 10993 is superseding USP as the reference standard in measuring material biocompatibility. USP protocols are used to classify plastics in Classes I - VI, based on end use, type and time of exposure of human tissue to plastics, of which Class VI requires the most stringent testing of all the six classes.
- Systemic toxicity tests are used to determine the irritant effect of toxic leachables present in extracts of test materials.
- Intracutaneous tests are used to assess the localised reaction of tissue to leachable substances.
- Implantation tests are used to evaluate the reaction of living tissue to the plastic.
STUDY TITLE: USP <88> “CLASS VI” Tests Acute Systemic Toxicity, Intracutaneous Irritation and Intramuscular Implantation.
Systemic Injection
Clinical Observations: None of the test or control animals exhibited overt signs of toxicity at any of the observation points. The test is considered negative because none of the animals injected with extracts of the test article showed a significantly greater biological reaction than the animals treated with the control articles.
Intracutaneous Injection Test
Clinical Observations: There were no overt signs of toxicity observed in any test or control animals. The difference between the test article and control article mean reaction scores (erythema/edema) was less than 1.0. The test article meets the requirements of the Intracutaneous Test.
Implant Test
Clinical Observations: There were no overt signs of toxicity noted in either animal. Macroscopic evaluation of the test and control article implant sites showed no significant infection, encapsulation, hemorrhage, necrosis, or discoloration. The test is considered negative, since in each rabbit the difference between the average scores for all of the categories of biological reaction for the test article and control article implant sites did not exceed 1.0, and the difference between the mean scores for all categories of biological reaction for all of the test article implant sites and the average score for all categories for all the control implant sites did not exceed 1.0. The test article meets the requirements of the Implantation Test.
Conclusion
The USP 0.9% Sodium Chloride for Injection (NaCl), 1 in 20 Ethanol in NaCl (EtOH), and Polyethylene Glycol 400 (PEG) and Cottonseed Oil (CSO) extracts of the test article and the test article, Silky Cup (Menstrual Cup), did not produce a biological response following intramuscular implantation in rabbits, intracutaneous injection in rabbits and systemic injection in mice. Therefore, the test article meets the requirements of the USP guidelines, for Class VI Plastics − 50 °C.
ROHS (Restriction of Hazardous Substances)
Silky Cup is not Non-Toxicity as per Directive 2011/65/EU Restriction of Hazardous Substances Directive (ROHS),
Conclusion
Based on the performed tests on selected part of submitted samples , the result of lead, mercury, cadmium, Hexavalent Chromium,_Polybrominated Biphenyls (PBB)), Polybrominated Diphenylethers (PBDEs) comply with the limits as set by by ROHS Directive 2011/65/EU Annex11: recasting 2002/95/EC
Crack / Flex Test
Repeated bending strain and flexing for crack growth test up to 1,50,000 cycles has been done for Silky Cup
Conclusion
No cut initiation up to 150kcs